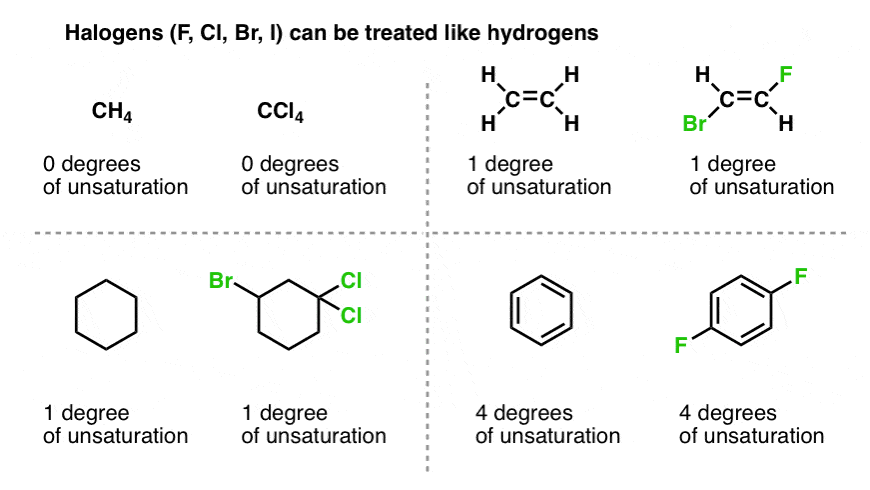
Any compound whose chemical formula has two hydrogens less than the maximum number possible 2n2 must have one ring or one double bond. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency
Hence the DoB formula divides by 2.

. In this molecule it has 3 double bonds and 1 ring so 3 1 4 degrees of unsaturation. Remove H s without changing the molecular weight. This has the effect of adding one degree of unsaturation.
Subtracted half the number of hydrogens and 5 for nitrogen 6-25 35 5 4. 2 Cl - Chlorine. The formula subtracts the number of Xs because a halogen X replaces a.
So a double negative charge is one degree of unsaturation. Benzene has 6 carbon atoms and 4 degrees of unsaturation 1 ring and 3 double bonds. The formula includes the number of hydrogen atoms added because of addition of nitrogen and subtracts the number of halogen atoms because they displace an equal number of.
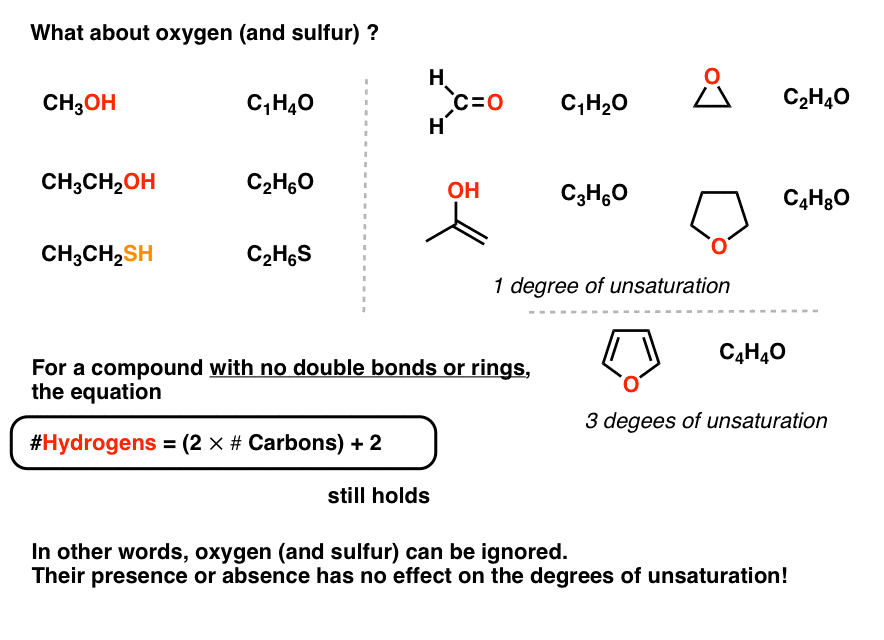
Radicals will always have n-and-a-half degrees of unsaturation where n is any integer including zero. As to the question as to why oxygen is dropped from the equation if the oxygen in hydroxychloroquine werent there the degree of unsaturation would still be the same. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
Degrees of unsaturation is equal to half the number of hydrogens the molecule needs to be fully saturated. The degree of unsaturation is equal to 2 or half the total number of hydrogen that a molecule needs to be classified as saturated. The Degree of Unsaturation is the rings plus the total number of multiple bonds.
Any compound whose chemical formula has two hydrogens less than the maximum number possible 2n2 must have one ring or one double bond. D e g u n s a t C 1 H h a l o g e n s N 2. I know you can C5H12 from Cn Hn2 and based on.
4 degrees of unsaturation means it can have any combination of double bonds or rings but has to add up to 4. 3 Br - Bromine. If youre referring to degrees of unsaturation as given by the formula.
Hence the DoB formula divides by 2. A If you have too few number of degrees of unsaturation you must add degrees of unsaturation ie. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
Benzene is a cyclic compound with one ring. This compound has 2 degrees of unsaturation 42 2. Begingroup In principle for any negative charge there is half a degree of unsaturation.
Then the answer is a qualified NO. The formula would indicate that this molecule has 15 degrees of unsaturation. Given a particular hydrocarbon structure for which you know the number of carbons and the degree of unsaturation you can calculate the number of hydrogen atoms.
However I think it is mostly applied to organic compounds. If it was a single negative charge that would be half a degree of unsaturation. The compound needs 4 more hydrogens in order to be fully saturated expected number of hydrogens-observed number of hydrogens8-44.
This also has the effect of adding one degree of. 1 F - Fluorine. Calculate the degree of unsaturation for benzene.
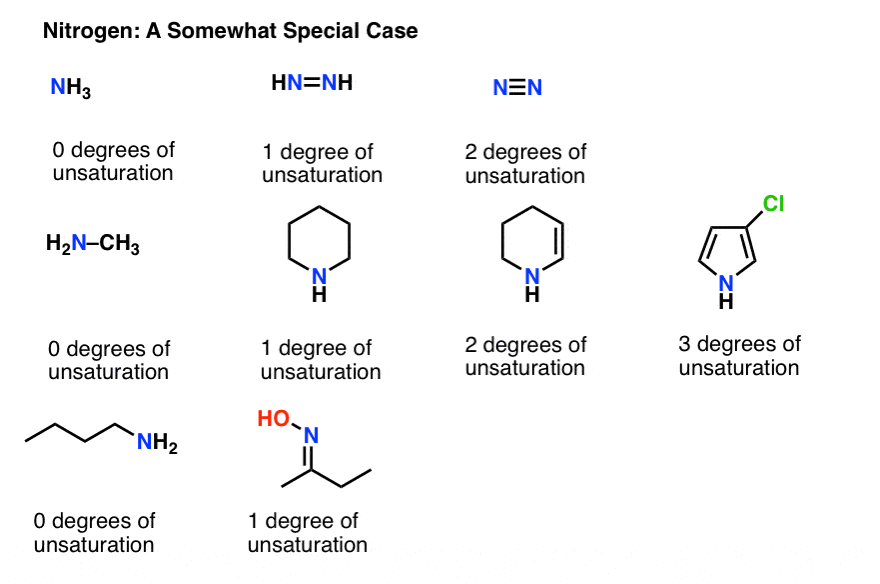
Hence the DoB formula divides by 2. 2 Replace C2H4 with N2. One for the double bond and one-half for the missing electron on nitrogen.
The degree of unsaturation is equal to 2 or half the total number of hydrogen that a molecule needs to be classified as saturated. The degree of unsaturation may also be employed in another way. 1 Replace CH4 with O.
Tap card to see definition. The formula is intended to tell you the total number of rings pi bonds which of course has to be a whole number. For example the Degrees of Unsaturation of tetrahydrofuran and cyclobutane are the same DU.
Hence the DoB formula divides by 2. 4 I - Iodine. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
Click again to see term. The notion of the degree of unsaturation of an organic compound is derived simply from the tetravalency of carbon. There are a few ways to do this.
Hence the degree of unsaturation formula divides the value by 2. The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound. The notion of the degree of unsaturation of an organic compound is derived simply from the tetravalency of carbon.
Hence the degree of unsaturation formula divides the value by 2. Click card to see definition. Tap again to see term.
The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound. Hence the DoB formula divides by 2. The DoU of compounds containing elements other than carbon and hydrogen can.
Can degree of unsaturation be a decimal. Can you have a half degree of unsaturation. Now divide 14 by 2.
Degrees of Unsaturation Formula. The Degree of Unsaturation is 7.
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency

0 Comments